Understanding Biotech: A Primer on Biologics
The History and Manufacturing Process behind Biopharmaceuticals
“Biology is the most powerful technology ever created. DNA is software, protein are hardware, cells are factories.” — Arvind Gupta
Coming off our primer on small molecule drugs, we learned that those medications are chemically synthesized into compounds that have some effect on our biological machinery when introduced to the body. As we come to biologics, we will learn that not only are we affecting our own biological machinery when they are introduced, but we are directly harnessing biological machinery for their production. Biologics, or biopharmaceuticals, are biological compounds that are derived from, produced by, or otherwise made of cells.
The creation of biologics is incredibly recent in comparison to the history of small molecule drugs. Advances in cellular biology, engineering, biochemistry, imaging and more were vital pre-requisites to biologics development. We’ll see in the history and the present of biologics manufacture that the harnessing of cellular machinery introduces more challenges than traditional drug manufacturing. Due to the size and complexity of biological compounds, the use of chemical synthesis is currently impossible. Since artificial chemical synthesis is off the table, we must rely on the source we already know works to create these compounds: living cells.
In the rest of this post we’ll:
Review the definitions of the molecules in this series
Dive into the history of insulin and insulin manufacture as the flagship biologic
Outline how biologics manufacture occurs today
Discuss new areas of innovation occurring in the industry
Definitions:
Small Molecule Drugs:
chemically synthesized molecules that are designed to interact with specific biological pathways in the body.
Some otherwise defined biologics like certain antibiotics are considered small molecule drugs because of their small size, relative simplicity to create, and major differences in their development processes from other biologics
Examples include aspirin, certain antibiotics, and statins
Biologics:
Substances that are derived from living organisms or their cells
In some cases biologics can be whole cells like in the case of engineered T cells
Other examples include hormones like insulin, antibodies, and vaccines
Nanotechnology:
The creation of materials or substances like nanoparticles that can perform functions on the 1–100 nanometer level in cells — like delivering particles into cells or repairing damage. For reference a DNA double helix is around 2nm in diameter and a water molecule is .275 nm
Examples include the nanoparticles that carry mRNA vaccines and other therapeutics into cells.
Insulin and Diabetes
Insulin aside from being the flagship use case of biologics is perhaps one of the most known pharmaceuticals today for its use in treating Diabetes Mellitus Type I and II. In normal functioning humans, the hormone insulin is secreted by the pancreas as a response to elevated glucose (sugar) levels in the blood in order to prompt changes in cells to counteract this elevation. The primary response is by triggering glucose uptake by muscle cells which account for 70–80% of insulin stimulated glucose uptake in the body. It does this by binding to a receptor on the muscle cell surface and activating a signal cascade in the cell that moves GLUT4 transporter proteins to the surface of the cell. When GLUT4 is exposed at the surface, it allows glucose to enter the cell.
In type I diabetes, the pancreas cannot produce insulin because the body’s immune system mistakenly kills the pancreas’s beta cells that produce it. In type II diabetes, the body’s cells have trouble responding to the signal of insulin to trigger glucose uptake due to several metabolic derangements in the body. These include increased presence of free fatty acids and inflammatory cytokines caused by too much fat tissue and mitochondrial dysfunction that ultimately inhibits and interferes with parts of the insulin signaling pathway. In serious cases of type II diabetes, insulin injections are required to override these inhibitory phenomena in order to lower blood sugar.
The primary cases of diabetes in the historical record relate to Type I Diabetes due to the primary causes of Type II Diabetes being a more modern phenomenon. Diabetes was initially noticed as a condition that caused excessive urination, thirst, and dehydration. The word diabetes was coined by the Greek physician Aretaeus of Cappadocia deriving from the Greek word for “siphon” referring to this loss of water. Later, the latin word mellitus, meaning “sugar sweet” was appended to diabetes by the English physician Thomas Willis. Although an odd diagnostic referring to the taste of the urine, it was an important revelation that sugar was appearing in the urine of these patients. This occurs in both Type I and II diabetics as the body will try and remove excess blood sugar by excreting it out in the urine. However, due to the process of osmosis, the increased concentration of glucose in the urine also pulls in excess water to balance out this high concentration. This is what causes the diabetic hallmarks of extreme dehydration and thirst, excessive urination, and presence of glucose in the urine.
For the vast majority of human history, there was no treatment for this condition and having it was a death sentence. This was made even more sad because type I diabetes usually arises in children and young adults. The prognosis for these patients was a gradual wasting away caused by the body’s inability to extract the sugar energy from the blood, requiring the breakdown of the body to fuel itself. This breakdown of alternatives like fatty acids causes the build up of acidic ketone bodies (Diabetic Ketoacidosis) damaging organs and leading to coma and death. Prior to insulin, the standard treatment for diabetes was a starvation diet extremely low in carbohydrates that allowed diabetics to live slightly longer than otherwise.
The History of Insulin:
The first step in solving any problem is understanding it. For diabetes this means understanding how and why it arises. The first breakthrough came in 1889 via an experiment by two German researchers: Okar Minkowski and Joseph von Mering. In this experiment, they removed the pancreas from dogs and found that dogs without a pancreas developed the same symptoms as diabetics. In 1921, insulin was finally isolated from the surgically removed pancreas of a dog and proven to reverse diabetic symptoms when re-injected into the dog. The successful isolation of insulin from animal pancreas and then the first successful treatment on a 14 year old diabetic patient named Leonard Thompson a year later sparked a huge interest in insulin production. In the US and Denmark within the companies of Eli Lilly and what became Novo Nordisk respectively, mass production of insulin took off to treat what was before a death sentence.
The issue however was that the only way that was known to “create” insulin was the same as what was used in the first experiment: grinding up an animal pancreas and then isolating the insulin from the resulting mush. The main difference in the mass production process was that now instead of using dog pancreas they used the leftovers from cows and pigs after slaughter. This crude way of extracting insulin was extremely inefficient. In fact it took 1000’s of cow and pig pancreas to recover enough insulin to treat one diabetic for one year. This put a hard cap on supply and made insulin a scarce commodity.
In 1978 however, the paradigm for insulin production and the entire field of biotechnology was changed forever. That year, scientists at the company Genentech in partnership with Eli Lilly successfully inserted the gene for human insulin into E. coli bacteria using recombinant DNA technology. The ability to add genes coding for a specific biological molecule to a cell that can be cultivated to a large colony and produce a large amount of product is what birthed the entire biologics industry. This is the paradigm that we fundamentally still operate in today.
Biologics Manufacturing: Insulin Case Study
Biologics manufacturing is done by living cells because the processes to construct these large and complex molecules are far too intricate to be done by the relatively simple chemical reactions that make up small molecule drug creation. Thus, it relies on the already configured nano machinery of cells to ensure that the product is properly built. The reliance on living cells however, introduces much complexity into the manufacturing process. In this section we will go through each stage of the biopharmaceutical manufacturing process, explain the goals and constraints, and compare/contrast the modern and historical process.
Stage 1: Upstream Operations
Upstream operations refers to everything before product harvest. This means cell line engineering and cell cultivation. In modern biologics manufacturing, manufacturers maintain specifically engineered cell lines for certain products. These cell lines are called master cell banks (MCB) and are cryogenically frozen to maintain their state. It is critically important to maintain them unaltered because biological systems mutate over time as DNA replication errors compound. The cells that ultimately become producers of the biologic are formed from a working cell bank (WCB) that is descended from the master cell bank. The types of cells chosen depend on multiple factors like how quick they replicate, if they secrete the product or not, and how expensive they are to cultivate. For insulin, genetically modified E. coli cells are the common cell type used but many other cells are used in biologics manufacture. Some examples are yeast cells (S. cerevisiaie), mammalian (chinese hamster ovary), and human (human embryonic kidney).
Once a WCB sample is selected, it needs to be scaled to around 10% of the bioreactor working volume to optimize for the cellular growth curve during fermentation. Since a cell bank vial initially contains around 1mL it must be run through a “seed train” of progressively larger bioreactors until it is the proper volume to inoculate the production bioreactor. If the bioreactor is 20,000L then the cell culture that ultimately starts the batch will be 2,000L.
The bioreactor itself has two requirements: enable aseptic operations and provide the optimal environment for cellular growth and product formation. This has implications for the design of the bioreactor. Traditional bioreactors are stainless steel because the material does not leach into the cell medium, it is easy to clean, and it will not degrade from the harsh cleaning chemicals or the temperature extremes. There is also an increasing interest in single-use bioreactors made of polymeric materials that eliminate the need for cleaning procedures. In the case of traditional re-usable reactors the design is such that the cleaning procedures can most effectively remove any and all contamination from both the prior cell batch and the cleaning chemicals themselves. Other fascinating features of bioreactors today is their ability to continuously monitor the medium environment and feed the cells past what the original medium would have allowed them to grow and mixers that will ensure that extra added nutrients are distributed to all cells. The mixing blades are meticulously designed to ensure an even distribution without damaging the cells and different designs are better for tougher or more fragile cells.
When cells have exhausted their medium of nutrients or a certain amount of time has passed, this triggers the end of this phase. Remember that we don’t want cells living for too long because genetic mutations might occur that alter the product.
Historical Process:
This phase in the early days of insulin was occupied with procuring and grinding up the pancreas of livestock
Stage 2: Cell Lysis & Product Harvest
Cell lysis is a required phase for all biologics that are produced as intracellular products. Insulin for example is produced as an intracellular product typically within E. coli cells while monoclonal antibodies produced from something like chinese hamster ovary cells are secreted into the cell culture medium. Secretion of product helps to simplify the entire process because it removes this step and makes filtration and purification steps easier. Because insulin is retained within the bacterial cells, they need to go through a process to split the cells and release it.
There are a variety of cell lysis techniques that depend on both the cell type used and the product being recovered. The major categories of cell lysis are mechanical and non-mechanical. Mechanical cell lysis aims to use physical force to disrupt cell structural integrity and are used more frequently in animal cells since they lack rigid cell walls like bacteria and yeast. Common methods for mechanical lysis include high-pressure homogenization where cells are forced through a small orifice at high pressure and bead milling where cells are ruptured by shaking them with glass or stainless steel beads. Non-mechanical cell lysis relies on chemical effects and is frequently used for bacteria. Examples include chemical and enzymatic treatment that break down cell walls and membranes and osmotic shock treatment which forces water into cells causing them to break. In the case of insulin production, E. coli cells are treated with enzymatic treatment (lysozyme) to break down their cell walls and chemical treatment with a surfactant like Triton X-100 to break down cell membranes.
Historical Process:
Since the pancreas cells were animal cells the mechanical grinding up of the tissue sufficed for product harvest
Stage 3: Filtration
The filtration or clarification stage is focused on the separation of liquid from solid components in the mixture thus far. This is important to remove unwanted materials because the purification steps require absence of solid particles. In biologics manufacturing there are 3 main techniques for separating solid and liquid: centrifugation, depth filtration, and tangential flow filtration. These are often either used together or by filtration techniques alone.
Centrifugation is aptly named after the centrifugal force that it harnesses to settle denser matter farther away from the center of rotation than less dense matter. In the case of a centrifuge, that means along the bottoms and sides of the centrifuge vessel. Centrifugation however, while effective at removing much of the large particulate in the slurry, does not remove enough to be used on its own. Depth filtration is typically used after centrifugation where the intermediate mixture is fed into filter modules using a filter of cellulose fibers and polymeric resin that adds a positive charge to the membrane. The liquid is pulled against the filter using a pressure differential and particulates that are either too big for the pores or chemically attracted to the membrane are captured. In cases where depth filtration is used in place of centrifugation, it is common to take more than one pass through.
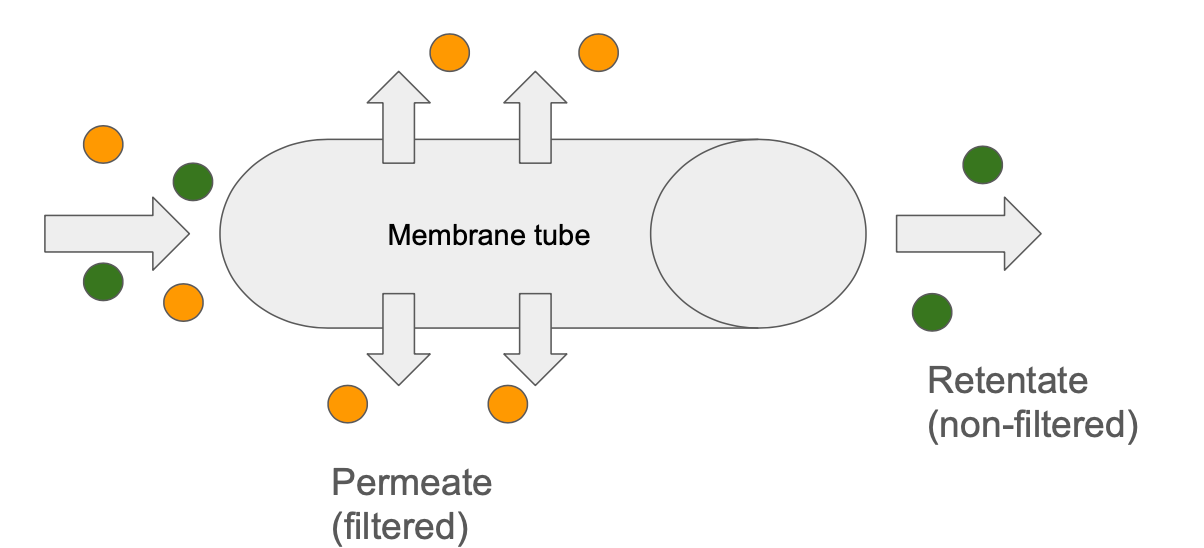
Tangential flow filtration (TFF) can also be used in place of the previous two and refers to a setup where the mixture travels through a membranous tube so that the flow of liquid is parallel to the membrane rather than directly against it in depth filtration. Pressure differentials encourage liquid to cross the membrane but because it is running parallel, it reduces solid build up in the membrane pores that prevents or slows down liquid perfusion. TFF is often used to prepare the product intermediate by concentrating it or exchanging the chemical buffer that it is in prior to purification steps.
In insulin manufacture today centrifugation is the first step after cell lysis because in this case, insulin itself is actually created initially within the bacteria as an insoluble inclusion body that is denser than much of the rest of the slurry. This means that after centrifugation, it is the solid insulin inclusion bodies that get extracted and move on in the process. After these inclusion bodies are extracted, they are solubilized and refolded in a buffer containing denaturants and other chemicals. From there filtration may occur to further clarify the solution, concentrate the insulin, or exchange the buffer before purification.
Historical Process
Insulin from pancreatic cells was already liquid so centrifugation operated in the reverse in that the liquid was retained and the solid cell and tissue mass was discarded. In contrast with today’s advanced cellulose and polymeric filters and pressurized systems, cloth and paper filters were used to separate more solid particles from the liquid by pouring it over the filter and letting gravity pull it through.
Stage 4: Purification
Biologics are unique drugs not only because they involve living cells, but also because of the way they are administered. Because of the chemical composition of these compounds, they cannot be taken orally or else they can be broken down or deactivated in the digestive system. Instead, these drugs need to be taken parenterally, meaning by infusion/injection. Because of this direct injection into the body, biologics have very stringent quality and safety standards. The purification stage is vital to ensuring these requirements.
After the clarification and filtration stage, the resulting intermediate is free of insoluble impurities. While a good first start, there are still many soluble impurities that have made it through and could seriously impact product safety and quality. Some examples are host cell proteins, endotoxins, viruses, and residual chemicals from upstream processes. Removing these is the purpose of the purification stage. Chromatography is the most popular technique for this stage and will thus be the focus here.
Chromatography is a chemical separation technique that was first used to separate plant pigments — where the technique got its name (chroma meaning color). It operates on the fact that through the uniqueness of the chemical structure of different molecules in a fluid mixture, they will pass through a given “filter” at different speeds. This is determined by their molecular interactions with the filter. The types of interactions needed will dictate the type of chromatography being used and could be based on chemical binding affinity, electrostatic binding based on charge of the molecule and filter, or size exclusion based on the size of the molecule. In chromatography, the mixture including the product is referred to as the mobile phase, and the “filter” that is packed within the column that the mixture flows through is called the stationary phase, or the resin.
It is very common to go through multiple passes of different types of chromatography to progressively filter out various types of impurities. In insulin for example, the process could go through three different types. Starting with ion exchange chromatography, when the mixture is poured through the resin, insulin and other similarly charged molecules will be electrostatically bound to the resin while other molecules considered impurities without charge affinity will flow through freely. Then a new chemical is poured in that changes the electrostatic environment of the column so that the insulin unbinds and flows through. After that, affinity chromatography will be used where the resin contains insulin specific ligands for the insulin to bind to while other non-specific impurities are washed away. Like the previous step, a new chemical will be introduced to break the bonds and allow for the insulin to be removed purely. Lastly, reverse phase chromatography may be used based on hydrophobic interactions. Insulin, containing hydrophobic regions will bind via hydrophobic interactions with the stationary phase while mis-folded insulin variants will wash through.
The end result of this process is both very pure in that it is free from non-insulin impurities but also of high quality in that it is free of improperly constructed insulin.
Historical Process:
This phase historically was a little different and relied more on crystallization of insulin. The steps include first precipitating insulin out using alcohol, then running it through ion-exchange chromatography (based on charge), and then again crystalizing it using zinc and washing the crystals. In early production of insulin based on animal insulin, it was too common by today’s standards to have immune reactions to animal host cell material not successfully removed.
Stage 5: Formulation
While the resulting product intermediate from the last phase of chromatography is very pure, it is also very likely to be at a different concentration and within a different buffer than is required for the end drug product. Formulation is the phase where the drug substance is fully prepped to be converted to drug product when ready. One technique you may probably already guess is coming is tangential flow filtration in order to adjust concentration and exchange buffer. This is indeed used but in a slight variation called ultrafiltration. The main difference is that the membrane is setup to retain the product while the solvent filters through, making it ideal for buffer and concentration adjustment.
It is important to note that the formulation phase ends the production stage of drug substance rather than drug product. The difference being that the drug product is that which is fully ready for medical use. It is common for the drug substance produced at the end of the formulation stage to include excipients that modify drug behavior as well as make it more stable for storage or transport. In the case of insulin, these may be preservatives to prevent microbial growth as well as modifiers that slow down insulin absorption in the body. This finished drug substance is bulk filled into a container for frozen or refrigerated storage. When the product is ready to be shipped, it is thawed, pooled, additionally compounded with excipients if required, and then filled into the final drug product container like a vial or syringe.
Historical process:
After the final purification step of crystallization, the crystals needed to be dissolved and compounded with excipients. This process contains many similarities with today in the types of excipients involved to stabilize and preserve it. The main differences were that historical insulin formulations did not have the same modifications that changed the absorption profile of insulin.
Innovations in Biologics Manufacture
As we see from the analysis of the previous case study, biologics manufacture has made enormous advancements in the technology we have brought to bear on it. Advancements in genetic engineering first allowed us to hijack cells like bacteria to produce human proteins for us rather than relying on harvesting small amounts from animals. Further advancements in genetic engineering have opened the doors both to more efficient target protein production but also new types of therapeutics like CAR T-cell therapy. This therapy modifies a patient’s own immune T-cells to attack a specific target. It has shown to be highly effective against blood cancers and new data shows a lot of promise for curing autoimmune diseases as well.
Advancements in machine technology and biochemistry have also allowed production to scale and reach incredible levels of purity and safety. Emerging in the field are new technology trends around:
Single-use equipment
This allows intense development of cleaning procedures to be bypassed because equipment is discarded after the batch.
Continuous manufacture
Like we discussed in small-molecule drug manufacture, there is also a focus on bringing continuous manufacturing principles to biologics. One example we briefly discussed was the modification of bioreactors to support continuous perfusion of nutrients to cell cultures in order to expand yield beyond the amount in the initial medium. These types of reactors are especially great for biologics that are secreted by the cells because the medium is continually harvested and replaced allowing for extended batch runs. These are ended after a predetermined time (potentially weeks or months later) in order to prevent cellular mutations that may affect drug quality. Automation around filtration and purification also makes the process of switching through different filters and chromatography types very efficient.
Closed and Automated Systems
Closed systems close off the manufacturing process from the open air greatly reducing contamination and making cleaning easier and more efficient. Automated systems work well with this and can integrate multiple steps of the manufacturing process without human intervention.
References:
Biopharmaceutical Manufacturing: Principles, Processes, and Practices: Gary Gilleski, Charles Rutter, Becky McCuen
Novo Nordisk (Ozempic): Acquired Podcast
History of Insulin: PMC3714061